Understanding Chlorine / Bromine
For Your Pool Or SpaChlorine / Bromine acts as a rapid and persistent sanitizer, an effective algaecide, and a strong oxidizer of undesired contaminants.
In determining chlorine in recreational water, the differentiation of free chlorine and total chlorine is needed.
- Free chlorine is the sum of hypochlorous acid and the hypochlorite ion. With pH control, hypochlorous acid, the sanitation work horse of chlorine, can be maximized in normal swimming pool conditions.
- Combined chlorine is a group of compounds formed by the reaction of free chlorine with inorganic nitrogen waste and other organic contaminants. Chloramines have little disinfecting capabilities relative to free chlorine, and are known to contribute to health issues with bathers. Total chlorine is the sum of both free and combined chlorine.
There are three ways to test for chlorine / bromine:
- OTO (Orthotolidine)
- DPD (Diethyl-P-Phenylenediamine), and
- FAS-DPD (Ferrous Ammonious Sulfate – Diethyl-P-Phenylenediamine)
Let’s look at each.
OTO (Orthotolidine)
OTO only reacts with total chlorine in your pool water sample, giving it a various shades of yellow depending on the concentration.
Check out the video to learn more.
DPD (Diethyl-P-Phenylenediamine)
DPD is the colourimetric version of the chlorine test, whereby free chlorine reacts with this indicator to produce a red colour.
When this indicator is added to the sample and free chlorine is present, a reaction occurs producing a red colour, and the intensity of the colour is compared to standardized colours to determine the amount of free chlorine.
Using the same sample, potassium iodide is then added and if combined chlorine is present the intensity of the red will increase and this would again be compared to the standardized colours to determine total chlorine. A subtraction calculation is used to determine combined chlorine.
FAS-DPD
(Ferrous Ammonious Sulfate – Diethyl-P-Phenylenediamine)
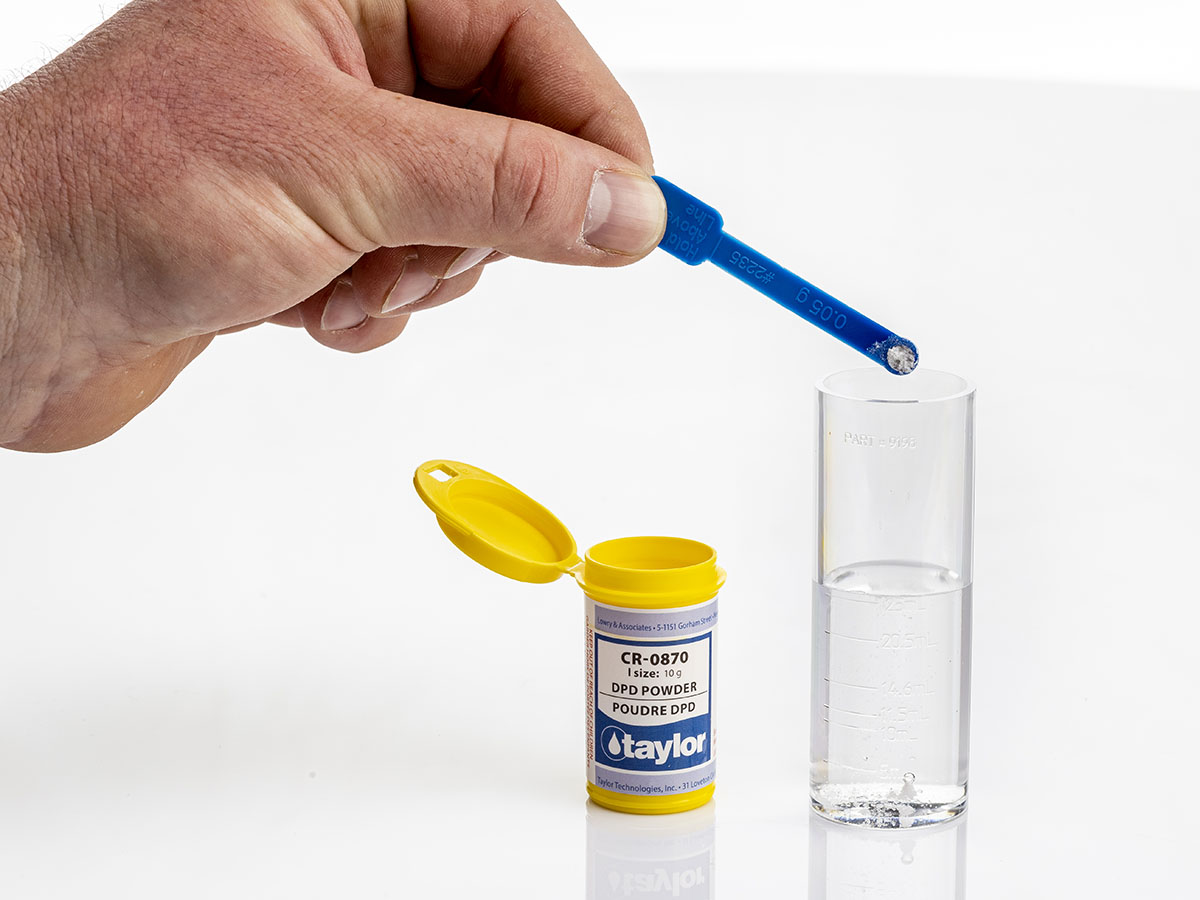
The titrimetric derivation of this test is called FAS-DPD. DPD is used as the indicator in this procedure, but ferrous ammonium sulphate (FAS) is the titrant used to determine free and combined chlorine.
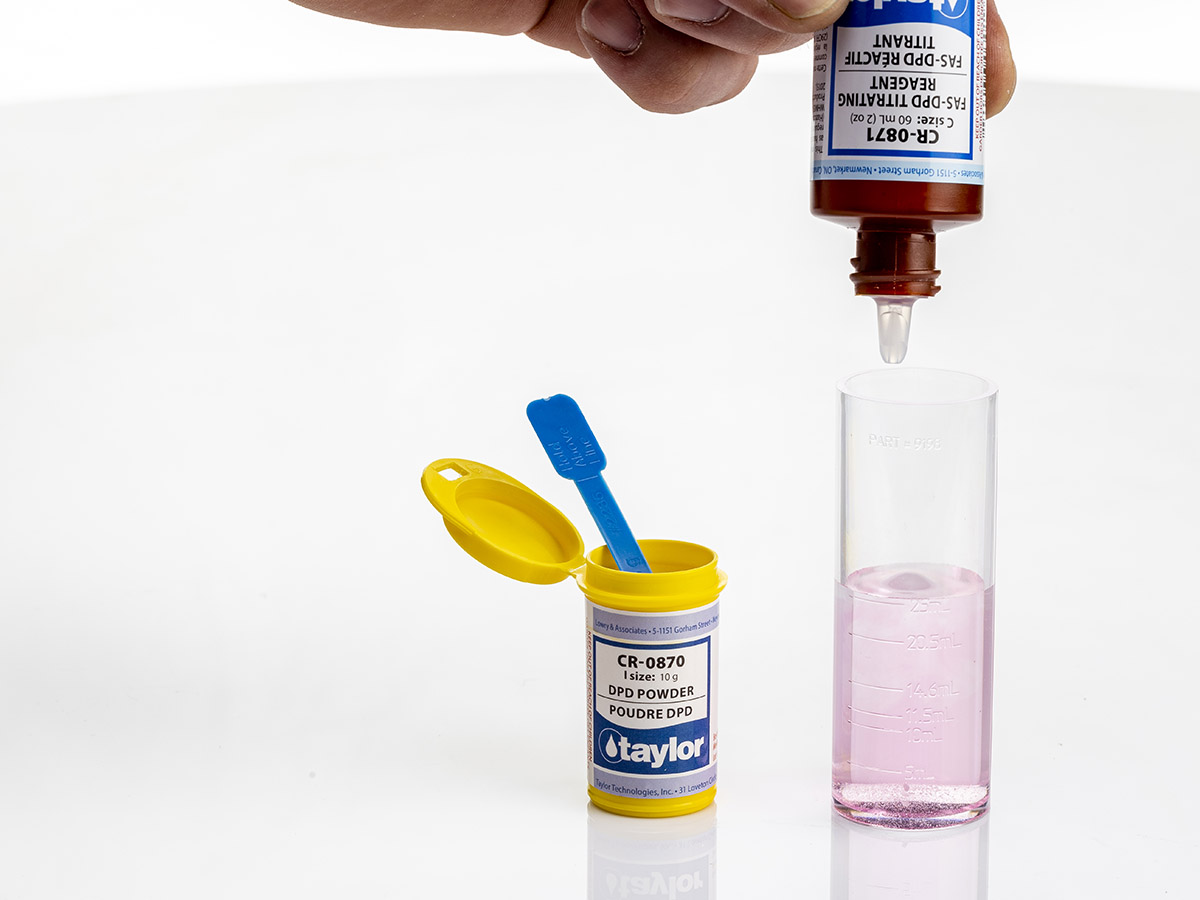
When the DPD indicator is added to the sample, just as DPD method mentioned earlier, the sample will turn red if free chlorine is present. The DPD indicator in this test is typically a powder and the sample size is smaller, but the same principle exists as the colourimetric DPD test.
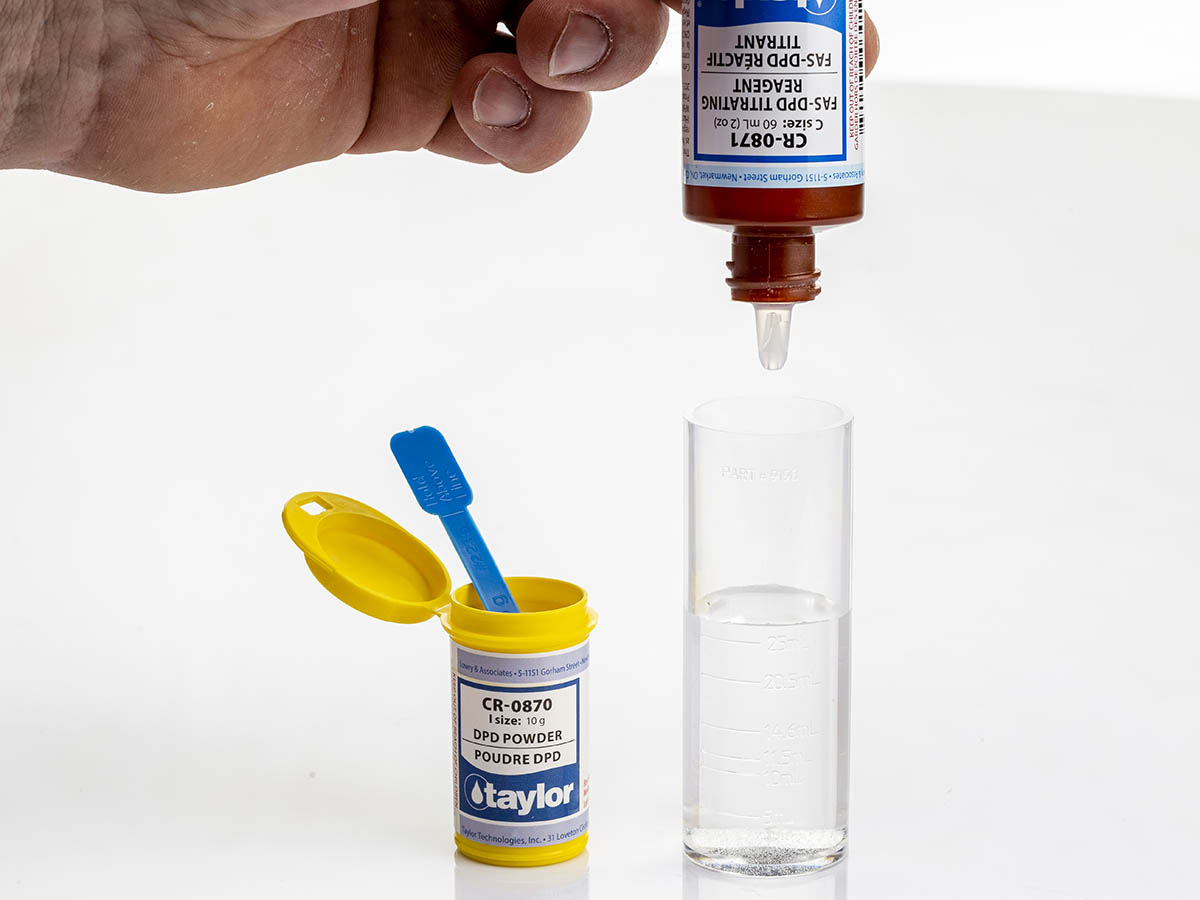
The FAS is a reducing agent, and as the titrant is added, the original red colour will disappear. The end point is the point where the red colour has completely disappeared.
Based on the amount of titrant added, the amount of free chlorine is determined. The subsequent potassium iodide is added and again if combined chlorine is present the sample will turn red. Titrate this sample with the FAS, and the amount needed to eliminate the red colour is the endpoint, and can be recorded as combined chlorine.